 |
|
|
|
|
|
|
 |
|
|
|
|
 |
Alkalized gelatin
and acidified gelatin are generally used.
However, when there is a possibility that
fill material may cause insolubility,
succinated gelatin is used. |
 |
 |
Adds elasticity to the capsule,
thus preventing cracking. Concentrated
glycerin and D-Sorbitol are mainly used. |
 |
 |
Ethyl paraben and propyl
paraben are mainly used. |
 |
 |
Titanium oxide is added
to light-sensitive compounds to prevent
light penetration. |
 |
 |
Colorants allow easy coloring
to make capsules more distinguishable
and appealing. |
 |
 |
Mixing with crystallized
gelatin prevents capsules from sticking
together or to a container, and also prevents
delayed dissolution of the capsules. |
 |
 |
Moisture content of the
capsule shells is reduced to 7 〜 9%. Generally,
moisture content that is too low could
lead to the tendency for cracking and
too high a moisture content could cause
problems such as sticking. |
 |
 |
Enteric coating enables
absorption in the intestinal tract. |
|
|
|
|
|
 |
 |
Drug
substances that are naturally in an oil
phase |
 |
 |
 |
Oil
phase drug substances that are diluted with
an oily base |
 |
 |
 |
Oil-soluble drug substances that are dissolved
in an oily base |
 |
 |
 |
Water-soluble
drug substances that are dissolved in an
aqueous base (Macrogol 400) |
 |
 |
 |
Drug
substances that are suspended in an oily
base |
|
 |
 |
 |
High gas barrier properties
of the capsule shell protect stability of
drug substances against oxidation |
 |
 |
Treatment of the capsule
shell with titanium oxide for protection
against light supports stability of drug
substances. |
 |
 |
Compounds that
are active at trace amounts, such as alfacalcidol,
can be injected uniformly on an μg scale.
|
 |
 |
Compounds that cannot be processed
into tablet form due to their relatively
low melting point can be filled as an oil
phase without melting into a soft capsule. |
 |
 |
Drug substances in oil phase
or as suspensions lead to higher bioavailability. |
 |
 |
Ability to formulate drug
substances with strong odor or volatile
compounds. |
 |
 |
Higher cost efficiency by
simplified manufacturing processes and high
product quality due to accurate and precise
encapsulation machinery. |
|
 |
 |
Vegetable
oils such as corn oil, soybean oil, sesame oil,
cottonseed oil, safflower oil, wheat germ oil,
and middle chain triglycerides have been widely
used.
Oily bases used especially for pharmaceutical
products are carefully selected based on multiple
studies such as drug substance stability. |
 |
 |
Macrogol
400 is used as an aqueous base. If a drug substance
is water soluble, it is either solubilized in
water first and mixed with Macrogol 400, or solubilized
directly in Macrogol 400. Excess water could lead
to problems after the encapsulation process. Drying
process conditions must be adjusted accordingly
when aqueous bases are used. |
 |
 |
When a drug substance
is solubilized in an oily base, it becomes clear.
However, when a drug substance is insoluble in
an oily base or its low solubility requires a
large volume of solution, then it is treated as
a suspension. Beeswax or surfactants are used
as a suspension agent for oily bases, and Macrogol
4000 or 6000 is used when Macrogol 400 is the
base. |
 |
 |
Surfactants are not
only used as a suspension agent, but also to enhance
solubility and stability. In addition, as an effect
on elution and absorption, surfactants are considered
to be important in the designing of inner fill
material formulation. Polysorbate, glycerin fatty
acid esters, and hydrogenated castor oil are mainly
used. |
 |
 |
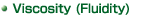 |
 |
 |
Clear Solublized
Solution - 2000 mm2/s or less (viscosity
rate)
Suspension Solution - 30000 mPa.s or less |
|
| |
 |
| |
 |
Particle
Size - Solid material should pass through
100 mesh |
|
| |
 |
| |
 |
Regular
range is 50 to 2000mg; however, amounts
beyond this range are also possible. |
|
|
 |
 |
| Sample production can be from as
small a scale as a couple kilograms to the manufacture
of investigational drugs by our Technical Research
Laboratory. During sample production, capsule
shell formulation, fill material formulation,
and data collection at critical steps of the manufacturing
process are performed. We design formulations
customized to the drug substances provided by
our customers. |
|
|
|
|
|
|
Stability testing is performed after the final
stage of bottling, PTP, or pillow packaging.
We also perform stability testing and elution
testing upon request.
|
|
|
|
|
|
|
|
|
|
 |
 |